Inspiration Can Pencil Lead Be Used As An Electrode, As a proof of concept application of the device we showed that the setup can be used.



Can pencil lead be used as an electrode.
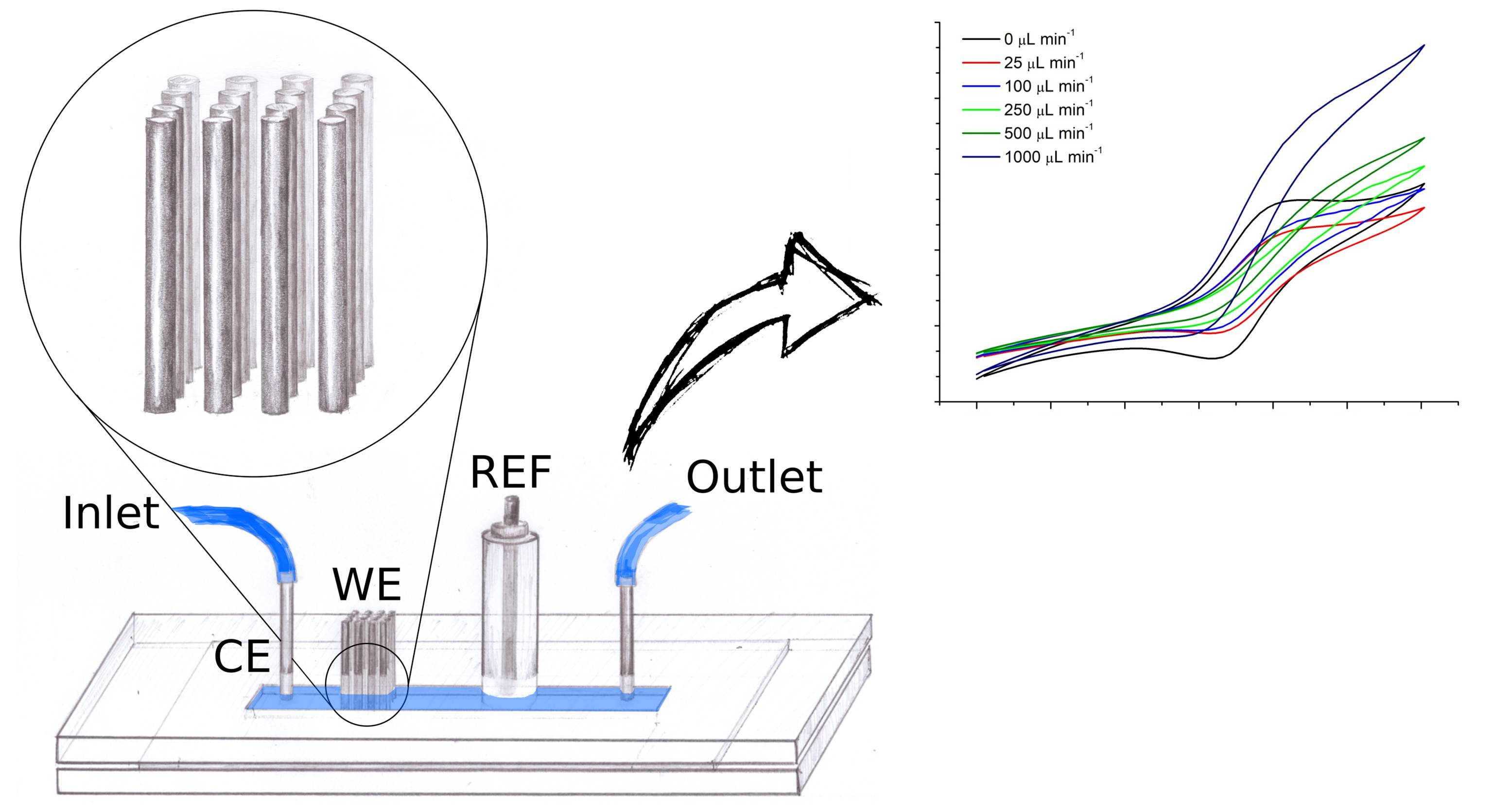
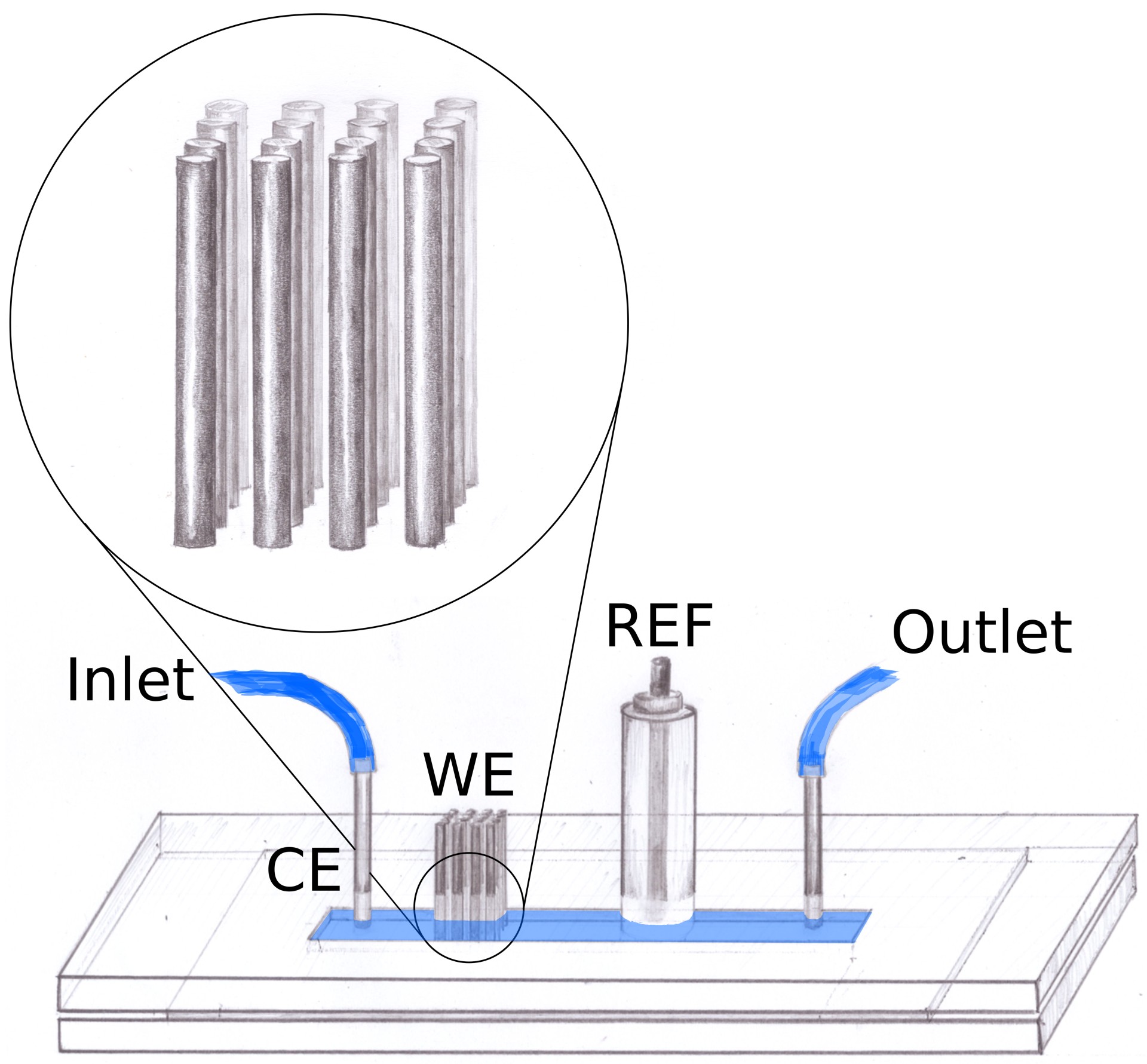
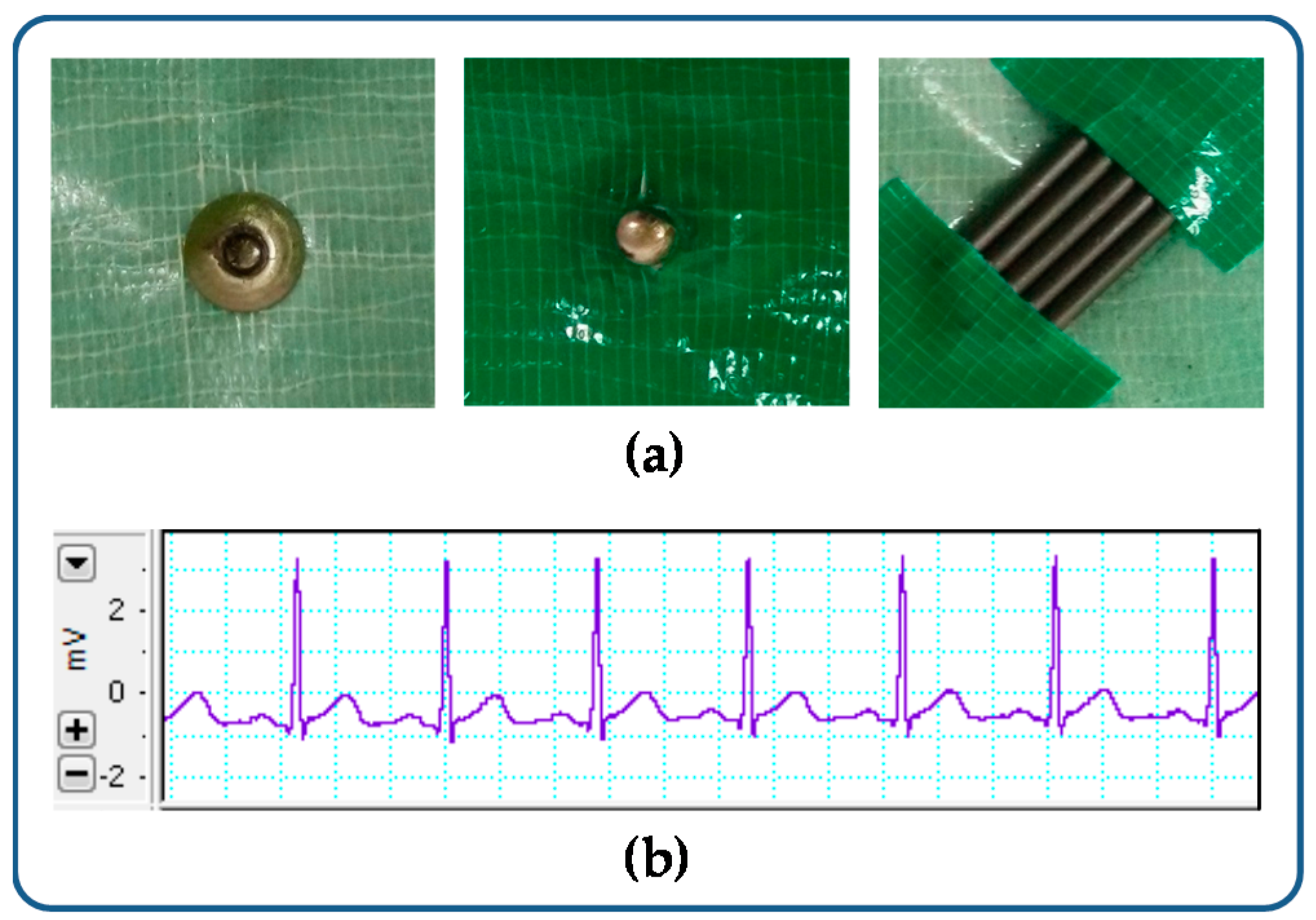
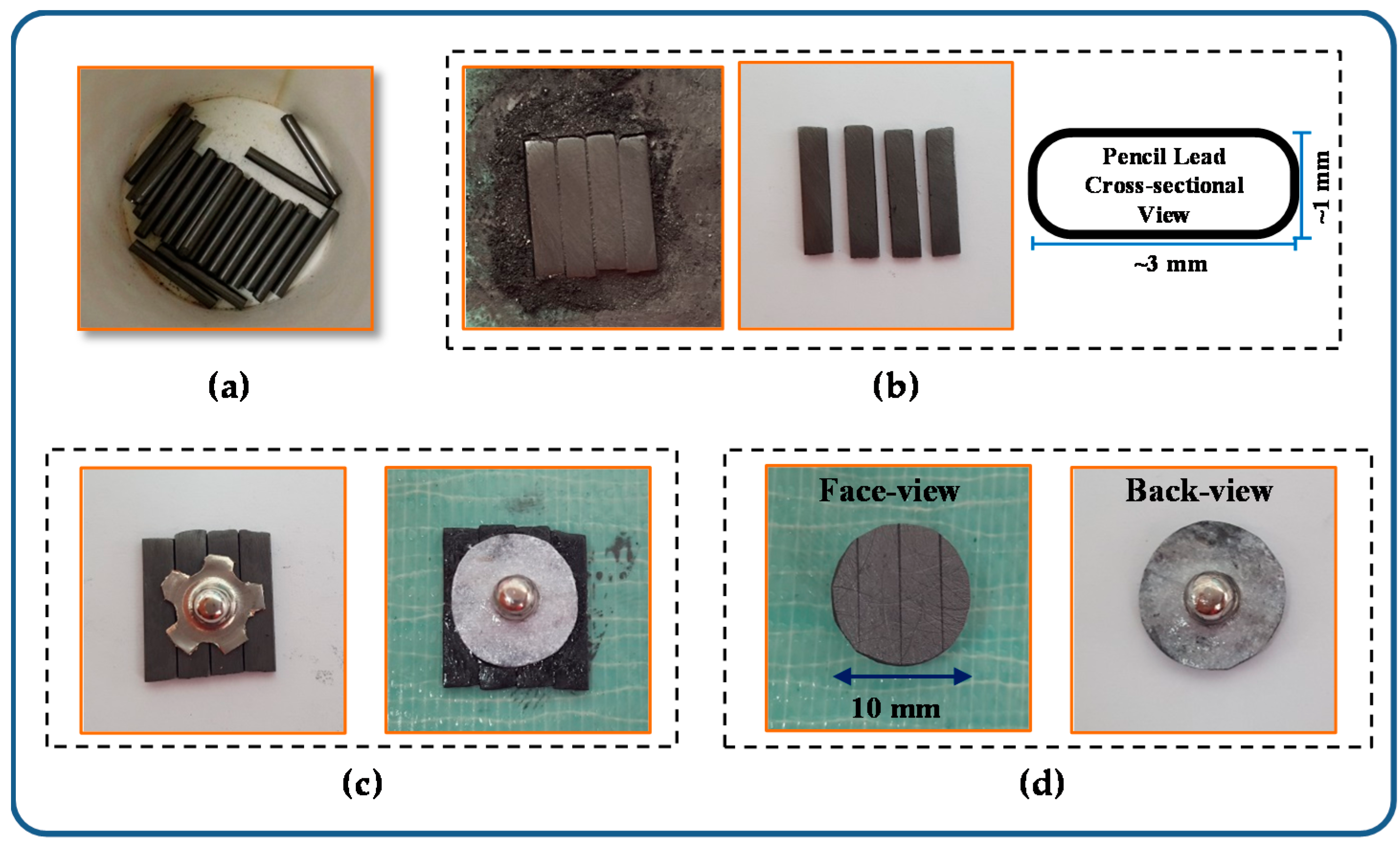
 The electrode array was integrated into a plexiglasspdms channel.
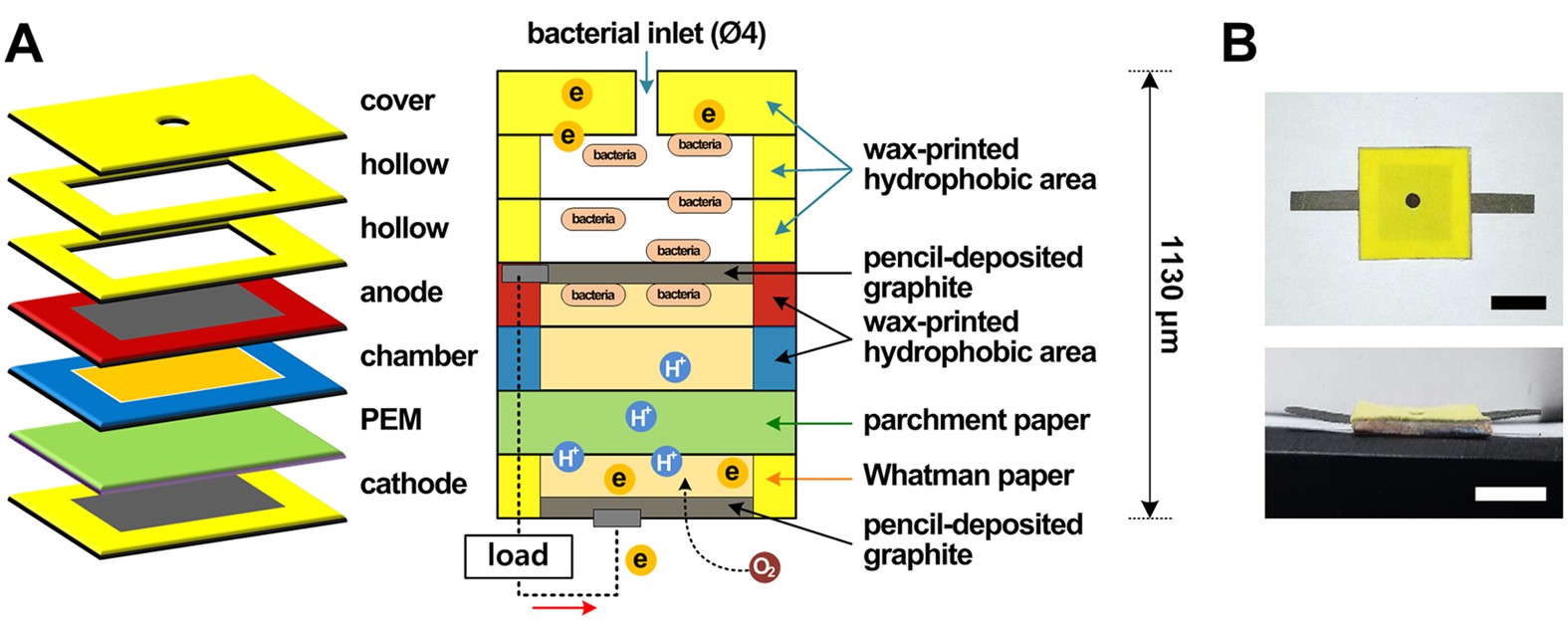
More recently pencil leads have been used to draw electrodes and conductive circuits in paper based sensors making these systems simpler inexpensive and solvent free.
Graphite is one of the few materials which is not a metal and which is a conductor.
Therefore the lead in pencils can be used as an electrode.
The electrode array was integrated into a plexiglasspdms channel.
More recently pencil leads have been used to draw electrodes and conductive circuits in paper based sensors making these systems simpler inexpensive and solvent free.
Graphite is one of the few materials which is not a metal and which is a conductor.
Therefore the lead in pencils can be used as an electrode.
 We tested the setup using a simple redox probe and compared the results with computer simulations.
This was a test to see if pencil lead can be used as an electrode material in electrochemical lead analysis.
My attempt and results.
I have a paper goldchamp et al j.
We tested the setup using a simple redox probe and compared the results with computer simulations.
This was a test to see if pencil lead can be used as an electrode material in electrochemical lead analysis.
My attempt and results.
I have a paper goldchamp et al j.
 Which pencil lead is best for making electrode.
I have used different types hb 2b 2h but i havent recorded a good signal in cyclic voltammetry.
The exact diameter of ainstein lead is 026 mm.
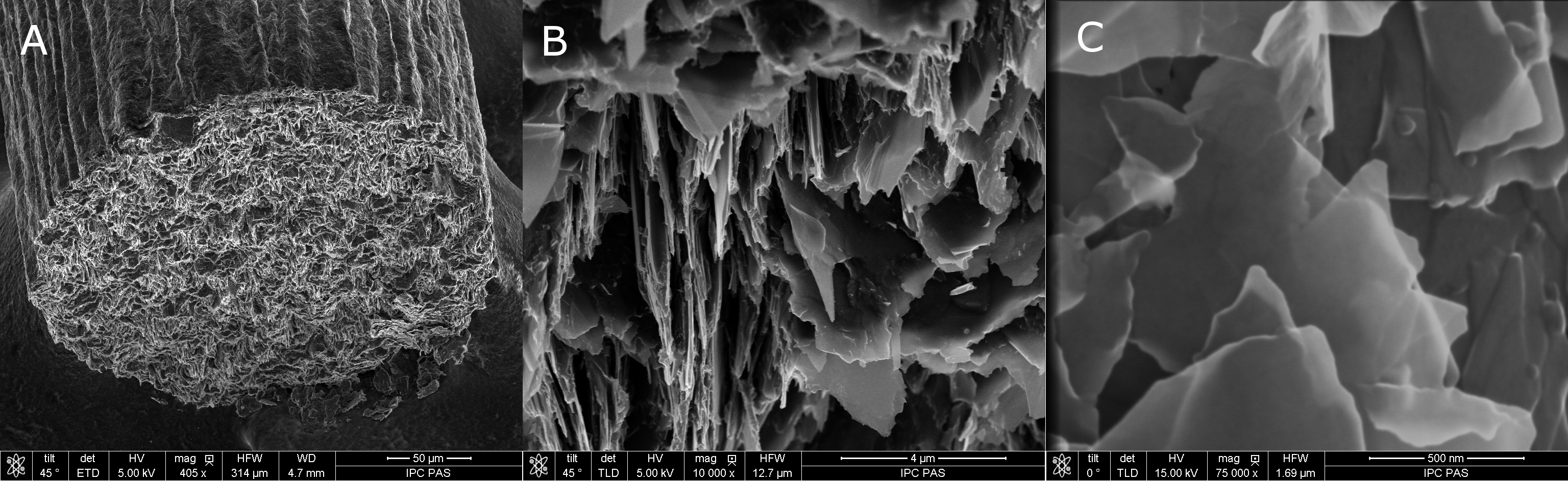
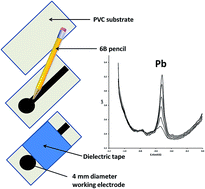
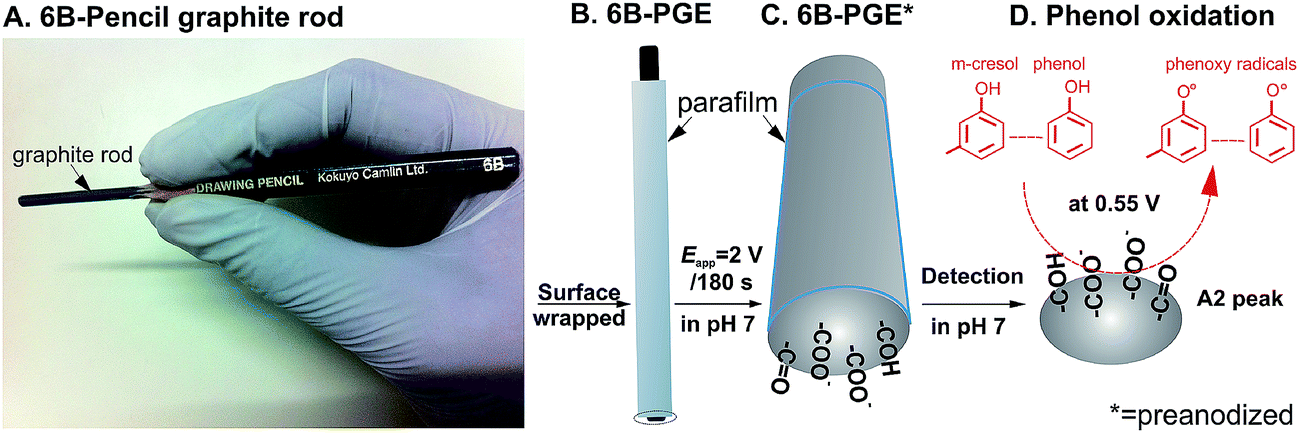
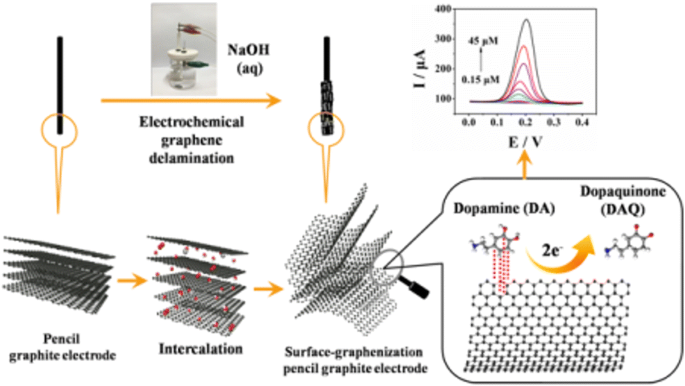
The electrode material of the pge is represented by a general purpose writing pencil offering the advantages of being user and environmental friendly it is nontoxic largely commercially available and simple to purchase at low costs eg in romania the graphite pencil lead used for one pge costs less than 004 being thus a disposable and readily renewable electrode.
Which pencil lead is best for making electrode.
I have used different types hb 2b 2h but i havent recorded a good signal in cyclic voltammetry.
The exact diameter of ainstein lead is 026 mm.
The electrode material of the pge is represented by a general purpose writing pencil offering the advantages of being user and environmental friendly it is nontoxic largely commercially available and simple to purchase at low costs eg in romania the graphite pencil lead used for one pge costs less than 004 being thus a disposable and readily renewable electrode.
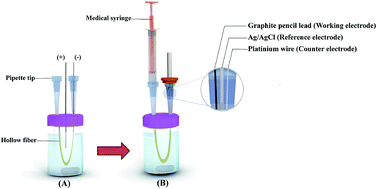
 I am not sure what a electrode is but i substituted a wire for pencil lead and it worked but it didnt work as well as a wire.
Graphite is a decent conductor of electricity.
I want to make a pencil lead electrode.
Pencil lead is not lead at all but graphite.
I am not sure what a electrode is but i substituted a wire for pencil lead and it worked but it didnt work as well as a wire.
Graphite is a decent conductor of electricity.
I want to make a pencil lead electrode.
Pencil lead is not lead at all but graphite.
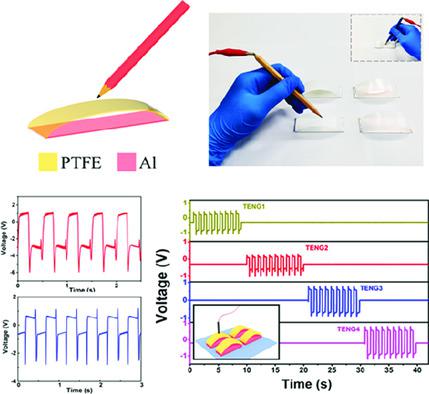
 Pencils make good simple electrodes for doing electrolysis or for testing conductivity.
Pencil leads have been increasingly used as electrode material in electrochemical applications.
But as far we know the application of pencil drawn electrode with enzymes is still limited or even non existing predicting a new window of opportunities in research.
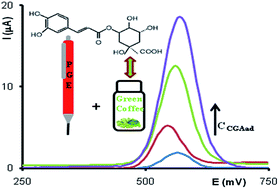
Ed 2008 85 976 that discusses using pencil leads as electrodes in the electrochemical determination of lead.
Pencils make good simple electrodes for doing electrolysis or for testing conductivity.
Pencil leads have been increasingly used as electrode material in electrochemical applications.
But as far we know the application of pencil drawn electrode with enzymes is still limited or even non existing predicting a new window of opportunities in research.
Ed 2008 85 976 that discusses using pencil leads as electrodes in the electrochemical determination of lead.
 In addition graphite is insoluble and nonreactive in aqueous solutions so we dont have to worry about it reacting or dissolving.
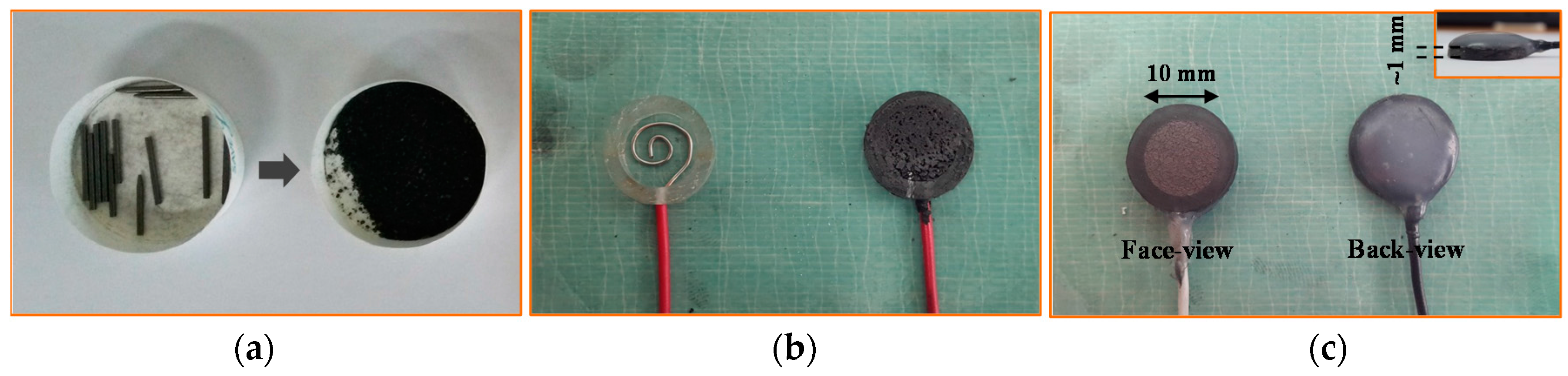
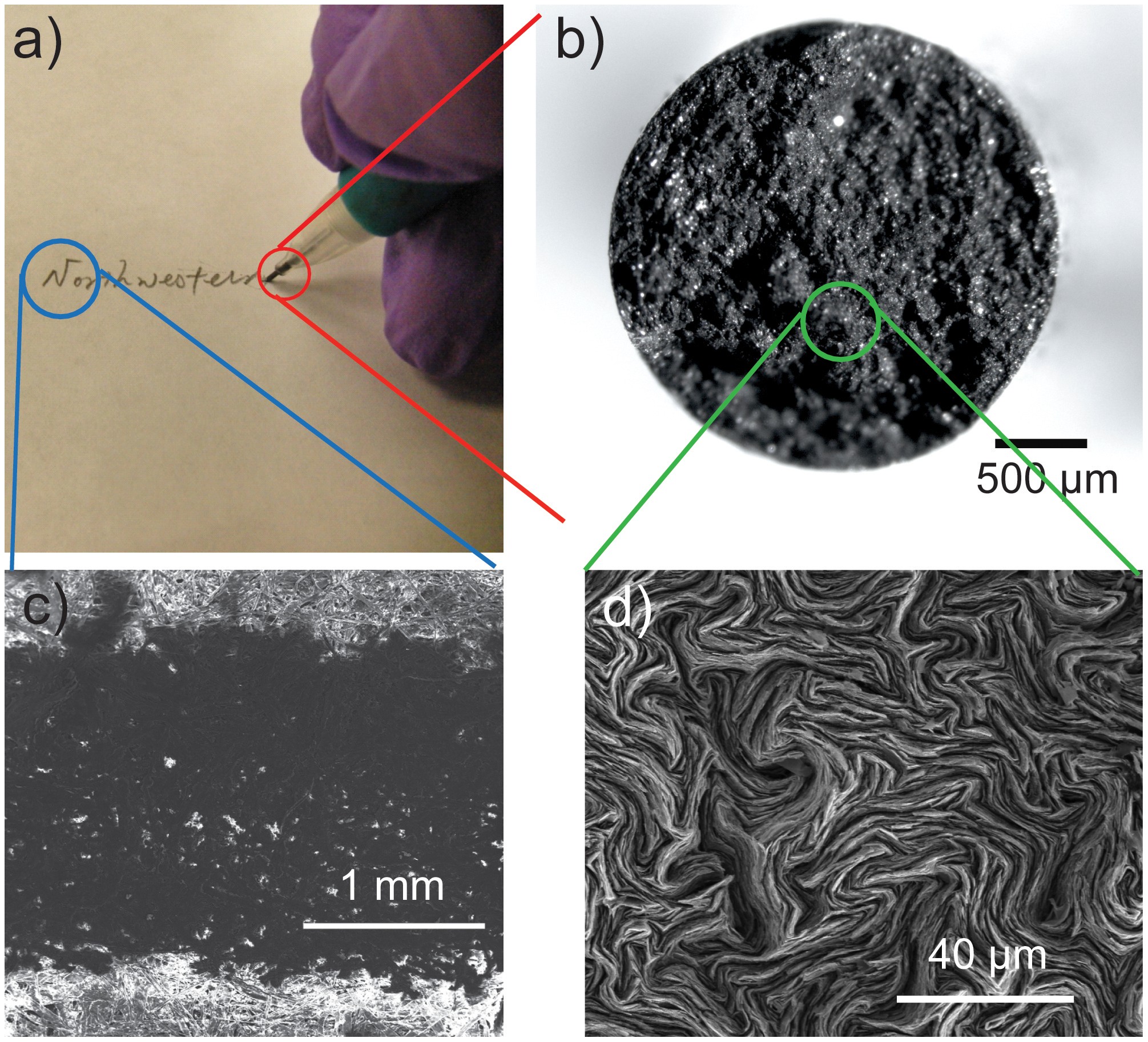
An ordered 3d array of pencil leads.
In addition graphite is insoluble and nonreactive in aqueous solutions so we dont have to worry about it reacting or dissolving.
An ordered 3d array of pencil leads.